- The Bottom Line on Telomeres and Longevity
- Key Insights on Telomeres and Longevity
- Why Telomeres Matter for Longevity and Healthy Ageing
- The Cell Cycle, DNA, and the Role of Telomere and Longevity
- Zombie Cells: Senescence, Telomeres and Longevity
- It’s not all about size
- How Inflammation, Stress and Environment Affect Telomeres and Longevity
- What is Telomerase and Why It Matters for Telomeres and Longevity
- Factors That Slow Telomere Shortening or Support Longevity Through Telomerase Activity
- Testing Telomere Length: What It Reveals About Longevity
- Telomerase Manipulation: A Double-Edged Sword
- Telo (Ending) Thoughts
The Bottom Line on Telomeres and Longevity
Telomeres are protective caps on chromosomes that shorten with each cell division, driving ageing and age-related disease. Inflammation, stress and environmental damage speed this process, while healthy lifestyle choices can slow it down. Telomerase offers limited repair, and testing telomere length may reveal a measure of ageing, but lifestyle optimisation remains the most effective strategy for longevity and healthy ageing.
Key Insights on Telomeres and Longevity
- Telomeres protect chromosomes and influence how cells age.
- Shortening telomeres contribute to senescence, chronic disease and reduced longevity.
- Stress, poor diet and environmental exposures accelerate telomere damage.
- Lifestyle choices such as movement, nutrition, sleep and stress management help preserve telomere health.
- Supporting telomeres is key to healthy ageing and long-term vitality.
Why Telomeres Matter for Longevity and Healthy Ageing
The word telomere comes from the Greek “telos” meaning “end” and “meros” meaning “part,” literally translating to “end part.” This fitting name describes the protective caps at the ends of our chromosomes, the intricate DNA structures that hold the blueprint of life.
Why are these tiny structures so important? Because they degrade. Each time a cell divides, telomeres get shorter. Over a lifetime, this small change impacts the systems that keep our cells, tissues and organs healthy.
This connection between telomeres and ageing is central to understanding longevity and healthspan. At Agami Health, we specialise in helping individuals explore and optimise the biological drivers of ageing and longevity. In this blog, The Truth About Telomeres and Longevity: Protecting Your Lifespan, we will share insights into how telomeres influence ageing, longevity and what this means for your long-term health and vitality.
The Cell Cycle, DNA, and the Role of Telomere and Longevity
Every cell in the body, except mature red blood cells, contains genetic information in the form of DNA (deoxyribonucleic acid). DNA is a beautiful molecule where two long strands of molecules called nucleotides form bonds with each other to create the shape of a ladder that then becomes twisted to form what’s called a double helix.
This double helix then winds into a super structure called a chromosome which is pictured below.
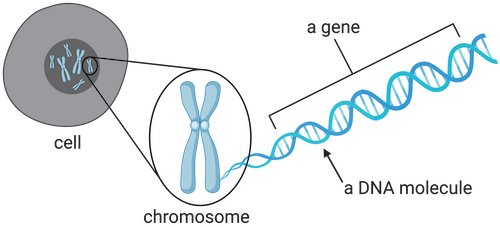
At both ends of each chromosome lies a telomere, the protective cap. These structures are central to the relationship between telomeres and longevity, as their gradual shortening over time influences how long our cells remain healthy and functional.
For humans to grow, and for old cells to be replaced by new ones, cells must divide from one into two in a process called mitosis. The goal of this process is to create an exact copy of the “parent” cell, which requires replication of the DNA within the parent cell to pass on to the “child.”
It turns out this process of copying DNA is flawed, in that the cell will lose a few bits of genetic code from the end of the strand of DNA each time it makes a copy. If we lost even a small amount of functional DNA in a cell, it would cease to be a functional cell and thus would either be harmful or useless, neither of which is desirable.
Amazingly, evolution has given us these telomeres to act as a buffer for this flawed DNA replication mechanism. Telomeres are actually non-functioning (non-coding) segments of DNA that we can afford to lose without any immediate consequence to the cell.
The key word here being, immediate. As you can imagine, after many cycles of this process, eventually you run out of spare telomere length to lose from the end of your chromosome. This is where the connection between telomeres and longevity becomes most evident, since the loss of almost the entire telomere length creates a critical moment at the cellular level from an ageing perspective.
The next cycle of cell division will then result in actually losing bits of critical DNA coding for functional components of the cell. When a cell reaches this point, it enters a state of cellular senescence. It will no longer divide even if needed.
This state of senescence however is more than just passively being asleep as the name may suggest. Aside from becoming dysfunctional or non-functional, cells in this state actively send all sorts of signals to surrounding cells triggering inflammation and other damaging downstream consequences.
Zombie Cells: Senescence, Telomeres and Longevity
Cells in the state of senescence are sometimes lovingly referred to as “zombie cells”. This is because they behave like zombies. They develop what is known as the senescence associated secretory phenotype, or SASP for short. They damage the surrounding cells and turn them into fellow zombie cells. However, like with all things in evolution, there is a reason for the existence of the process of senescence.
When transient and on a small scale, it is designed to prevent abnormal cells from dividing, signal to the immune system to clear up damaged or abnormal cells, and allow them to be replaced with new healthy ones (tissue regeneration). This process is critical for preventing tumours from dividing and growing as well as for repairing tissues after injury.
When this process becomes chronic and the burden of zombie cells becomes too great, then the result is detrimental. They release factors promoting inflammation, abnormal remodelling of the tissues around them and metabolic dysfunction such as insulin resistance and cardiovascular disease. The accumulation of senescent cells and telomere shortening are two of the strongest biological hallmarks of ageing. At our longevity clinic, we help individuals understand and act on these mechanisms with tailored approaches to cellular health.
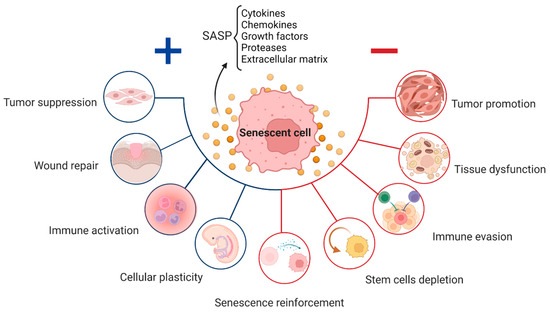
The result is marked increase in risk of numerous conditions including cardiovascular diseases (atherosclerosis, heart failure), neurodegenerative diseases (Alzheimer’s, Parkinson’s), metabolic diseases (diabetes, metabolic syndrome), musculoskeletal disorders (osteoporosis, osteoarthritis), fibrotic diseases (cirrhosis, idiopathic pulmonary fibrosis), and cancer.
It’s not all about size
Beyond the natural shortening that occurs with each cell division, telomeres can also be damaged by external and internal stressors. These influences, from chronic inflammation to environmental toxins, accelerate ageing at the cellular level and raise the risk of multiple diseases.
How Inflammation, Stress and Environment Affect Telomeres and Longevity
Chronic Inflammation
Inflammation is a double-edged sword. When acute, it’s part of the body’s defense and repair system, but when low-level inflammation simmers for years, the constant release of immune chemicals can accelerate telomere attrition. Think of it as a smoldering fire slowly charring the protective ends of DNA. This “inflammaging” state is strongly tied to chronic disease and is one of the key reasons why lifestyle choices that reduce inflammation, good nutrition, movement, stress control, play a central role in healthy aging.
Oxidative Stress
Telomeres are especially vulnerable to damage from free radicals, the unstable molecules produced by normal metabolism but amplified by things like pollution, poor diet, smoking, and even excessive exercise. Their high guanine content makes them prime targets for oxidative attack, which causes telomeres to shorten more quickly than they would from replication alone. Antioxidant defenses, from both the diet and the body’s own repair systems, act like shields, and when those shields are strong, telomeres are better protected.
Psychological Stress
Emotional stress may not seem like something that can change the structure of DNA, but the evidence is striking. Chronic psychological stress, whether from caregiving, financial strain, or persistent anxiety, has been linked to shorter telomeres and lower telomerase activity.
Stress hormones like cortisol fuel inflammation and oxidative damage, both of which erode telomeres. This is one reason why mindfulness practices, therapy, and resilience-building aren’t just “soft” tools, but real levers for protecting cellular health.
Environmental Exposures
Smoking, pollutants, and nutrition, external insults can directly or indirectly damage telomeres. Smoking introduces a cocktail of oxidative agents that directly chew away at DNA ends. Pollutants in the air, water, and food generate systemic inflammation. Poor nutrition, especially diets high in sugar and ultra-processed foods, deprive the body of protective micronutrients while fueling metabolic stress. On the flip side, diets rich in colourful plant foods, omega-3 fats, and other anti-inflammatory components have consistently been associated with longer telomeres.
What is Telomerase and Why It Matters for Telomeres and Longevity
In biology, an enzyme is a protein that speeds up a certain reaction or process. Inside cells, there is an enzyme called telomerase that actually adds extra DNA fragments to the ends of telomeres to try to counteract their natural breakdown. There is therefore a lifelong tug-of-war between telomere shortening and lengthening, with telomerase ultimately always losing to the inevitable drivers of shortening over time.
The question always comes back to why. Why does telomerase not solve the problem of telomere shortening? As with most things in aging, there are connections between hundreds of different pathways at play here.
Telomerase doesn’t fully compensate for telomere shortening over a lifetime for several key reasons:
- Limited expression in most cells
In humans, telomerase is not active in the majority of somatic (body) cells. It’s mainly active in stem cells and certain immune cells. Most of your tissues run on cells that divide without telomerase, so their telomeres inexorably shorten with each division. This is thought to be a protective mechanism against unchecked cell growth (i.e. cancer).
- Insufficient activity even in stem cells
Even where telomerase is active, it does not always fully restore telomere length. Stem cells gradually lose telomeres too, just more slowly. Over decades, this cumulative attrition still outpaces telomerase’s ability to “top up” telomeres.
- Oxidative stress accelerates shortening
Telomere DNA is especially sensitive to oxidative damage because of its high guanine content. Oxidative stress, inflammation, and metabolic factors can cause telomeres to erode faster than normal replication alone would cause, and telomerase cannot fully repair oxidative breaks.
- Cellular regulation against cancer
The body deliberately keeps telomerase in check. If telomerase were freely active in all cells, it would remove the replication limit that normally helps suppress malignant transformation. So, part of the reason it does not compensate is because evolution has “decided” that cancer risk is worse than gradual ageing.
- Heterogeneity of telomere maintenance
Even within the same tissue, some cells may have shorter telomeres than others. Telomerase preferentially elongates the shortest telomeres, but it does not do this uniformly or perfectly, leading to progressive loss at the population level.
So, in essence: telomerase is more of a partial safety net than a full repair system. It preserves the most crucial cell pools (like stem cells and germ cells), but doesn’t prevent the slow drift toward telomere depletion in the majority of tissues.
It is essentially a cancer-protection mechanism: if every cell could endlessly repair its telomeres, they could also divide without limit, which is exactly what cancer cells exploit.
Most cancers find ways to reactivate telomerase as part of their survival strategy. The trade-off, therefore, is that by restricting telomerase, we reduce cancer risk but pay the price with gradual telomere shortening and cellular aging.
Factors That Slow Telomere Shortening or Support Longevity Through Telomerase Activity
Several lifestyle factors have been shown to protect telomeres:
- Physical activity: Moderate and sustained exercise is linked to longer telomeres.
- Stress management: Practices such as meditation and yoga have been shown to boost telomerase activity in immune cells.
- Nutrition: Omega-3 fatty acids, vitamin D, polyphenols from colorful fruits and vegetables, and diets low in processed foods are all associated with healthier telomeres.
- Social connection and psychological wellbeing: Loneliness and social isolation correlate with shorter telomeres, while supportive relationships appear protective.
- Sleep quality: Regular, restorative sleep has a positive impact on telomere maintenance.
Lifestyle interventions such as nutrition, exercise, and stress management can make a measurable difference to telomere health. Our longevity clinic supports people in applying these strategies in a personalised way to protect long-term vitality.
Testing Telomere Length: What It Reveals About Longevity
The idea of measuring telomere length to estimate “biological age” has attracted interest. Several commercial labs now offer blood-based tests measuring average telomere length in white blood cells. While these can give a rough indication, there are important limitations:
- Variability between cells and tissues: Telomere length is not uniform across all cells in the body.
- Population averages vs. individual meaning: Very short telomeres correlate with increased disease risk and mortality, but predictive power for an individual is weaker.
- Dynamic nature: Telomeres can shorten or lengthen over time depending on lifestyle and environmental inputs.
Telomerase Manipulation: A Double-Edged Sword
There is research actively exploring the use of pharmacological agents that may preserve or extend telomere length:
- Pharmacological agents: Compounds like TA-65 (from Astragalus membranaceus) show modest telomere effects, though evidence is mixed.
- Experimental gene therapies: In animal models, direct telomerase gene therapy extends lifespan and reverses age-related changes, though human applications remain experimental.
Artificially boosting telomerase is alluring but comes with serious caveats. On the one hand, increasing telomerase activity could, in theory, rejuvenate cells, extend their lifespan, and protect against tissue decline. On the other hand, the same mechanism could rescue abnormal or pre-cancerous cells, giving them the ability to divide indefinitely. For now, the safer and more effective path is lifestyle strategies that gently modulate telomerase and reduce telomere erosion without risking unchecked cellular growth.
Telo (Ending) Thoughts
Telomeres act as both guardians and storytellers of our cellular history. Each shortening sequence reflects years of environmental exposures, lifestyle habits, and cellular stress. While we cannot stop time, we can influence how gracefully our cells age by protecting these delicate structures. Movement, nourishment, rest, and stress management leave a lasting imprint on our DNA. Emerging research on targeted therapies and natural compounds suggests that supporting telomere health may become central to personalised longevity strategies.
If you are ready to translate telomere science into actionable steps for healthy ageing, our longevity clinic provides expert guidance and membership options tailored to your personal health journey. Book a discovery call with Agami Health today to learn how telomeres and longevity influence your health, vitality and how we can support you on your longevity journey.