If you cut an apple in half and leave it on your kitchen counter, what happens? Within minutes, the pristine white flesh turns brown. It’s a simple chemical reaction: oxygen in the air reacts with the fruit. Now, imagine that same process happening inside your cells. This is the simplest way to visualize Oxidative Stress.
It is, in essence, biological rusting. While you can’t see it happening in the mirror like you can on the apple, it is considered one of the primary drivers of the aging process and chronic disease.
But before you rush to buy every antioxidant on the shelf, we need to pause. Biology is rarely black and white. As it turns out, a little bit of rust might actually be necessary to keep the machine running.
In this deep dive, we are going to move beyond the surface-level advice of “eat more blueberries.” We will explore the complex biology of the spark, the specific ways this “rust” drives diseases like Alzheimer’s and heart disease, and the precision medicine protocols we use at Agami Health to measure and manage it.
The Imperfect Engine: The Biology of the Spark

To understand oxidative stress, we have to look at the engines of our cells: the mitochondria.
Your mitochondria are responsible for turning the food you eat and the oxygen you breathe into energy (ATP). They do this via a complex assembly line called the Electron Transport Chain. In a perfect world, electrons would flow down this chain smoothly to create energy, with water as the only byproduct.
But biology is not perfect. It’s a bit like a combustion engine; even the cleanest Ferrari produces some exhaust fumes.
In the mitochondria, a small percentage of electrons (about 0.1–2%) “leak” out of the chain prematurely. These rogue electrons bind to oxygen to create Superoxide, a type of Reactive Oxygen Species (ROS).
Why did we evolve with a leaky engine?
It seems like a design flaw, doesn’t it? Why would nature build us with engines that slowly poison us?
The answer likely lies in a concept called Antagonistic Pleiotropy. Evolution doesn’t care if you live to be 100; it cares if you survive long enough to reproduce. High-performance energy production was prioritized for survival and growth in our youth, even if the “exhaust fumes” cause damage decades later.
Friend or Foe? The Nuance of ROS
For years, the prevailing wisdom was that ROS were universally bad. This led to the “Free Radical Theory of Aging”—the idea that if we could just scrub all free radicals from the body, we would live forever.
We now know it’s not that simple.
In the right context, these free radicals act as vital signalling molecules. They are the smoke alarm that tells the cell, “We are under stress! Build stronger defenses!”.
This role as a biological messenger is critical for adaptation. When ROS levels rise transiently—like during a sprint or a session in a sauna—they act as a distress flare that triggers powerful genetic switches. For instance, ROS stabilize specific protein sensors that signal the nucleus to activate defense pathways. Instead of just causing damage, that brief pulse of oxidative stress forces your cells to upgrade their entire defense system, producing more of their own protective enzymes to handle future challenges more effectively.
We see this mechanism most clearly in exercise. Physical exertion causes a temporary oxygen deficit and a naturally occurring burst of ROS production. Far from being a mistake, this specific signal switches on regulators like PGC-1α, which instructs your muscle cells to build more mitochondria and expand their capillary networks. This is why the “stress” of a workout actually makes you more resilient in the long run—it forces the body to adapt to handle the stress better next time.
This is the principle of Hormesis: a biological phenomenon where a mild stressor stimulates the body to adapt and grow stronger. If you completely blunt this signal—for example, by taking high doses of potent antioxidants immediately after a workout—you may actually prevent the health benefits of the exercise. You kill the messenger, so the body never adapts.
When the Balance Tips: The Clinical Consequences
The problem arises when the “exhaust fumes” (ROS) overwhelm the “cleaning crew” (your antioxidant defense system). This state of imbalance is Oxidative Stress.
When free radicals run rampant without being neutralized, they start stealing electrons from stable molecules. This causes a chain reaction of damage that affects the three most critical components of your biology: your DNA, your fats (lipids), and your proteins.
Here is how that cellular damage translates into the specific diseases we fear most.
1. Cardiovascular Disease: The Initiating Spark
For decades, people were told that LDL cholesterol is the “bad guy.” I have written previously about how cholesterol is neither “good” or “bad”, it is the particle carrying the cholesterol that can be labelled “good” or “bad”. These particles as you should know if you read our articles, is called an apoB particle.
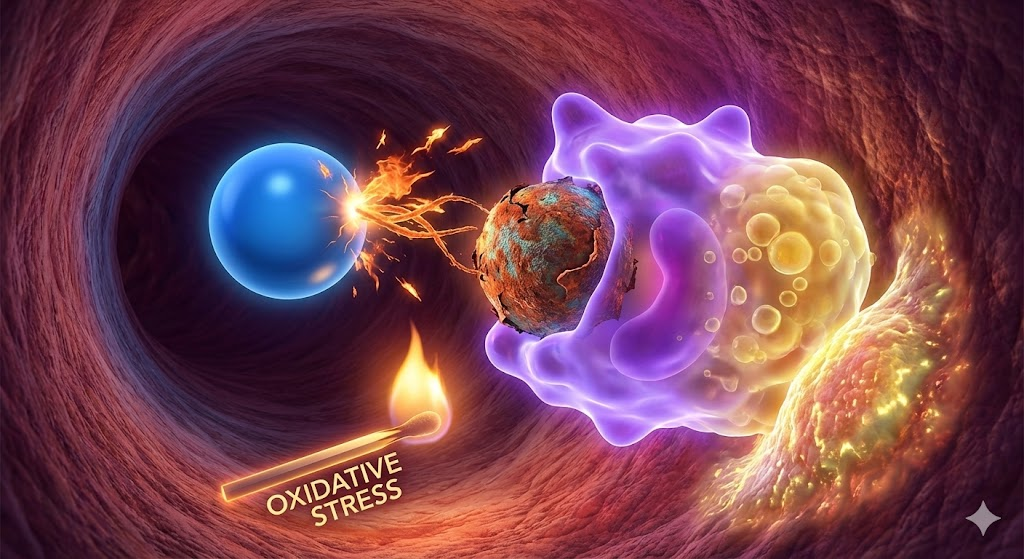
One of the first things that occurs in the process of atherosclerosis (clogging up of your arteries) after the apoB particle enters the blood vessel wall, is it becomes oxidised. Your immune system does not recognize this rusted cargo ship. Macrophages (immune cells) attack it, consuming the oxidized fat until they become bloated “foam cells.” These foam cells get stuck in your artery walls, forming the plaque that leads to heart attacks and strokes.
In this view, oxidative stress is the match that lights the fire of heart disease.
2. Neurodegeneration: The Brain on Fire
Your brain is particularly vulnerable to oxidative stress for two reasons. First, it is an energy hog—it consumes 20% of your body’s oxygen, meaning it has a massive mitochondrial load. Second, it is made of fat.
The brain is rich in polyunsaturated fatty acids, which are highly susceptible to lipid peroxidation (turning rancid). When oxidative stress hits the brain, it damages neurons and impairs the mitochondria that power your thoughts.
Mechanistically, oxidative stress promotes the misfolding of proteins—like the beta-amyloid and tau proteins seen in Alzheimer’s disease. It also disrupts the blood-brain barrier, allowing toxins to enter and inflammation to simmer. This is often experienced as “brain fog” in the early stages but can progress to significant cognitive decline over decades.
3. Metabolic Dysfunction & Diabetes
The beta-cells in your pancreas (which produce insulin) are exceptionally weak when it comes to antioxidant defenses. They have very low levels of the enzymes needed to neutralize free radicals.
When you have chronically high blood sugar, your mitochondria are forced to burn fuel at a rate they can’t handle, spewing out massive amounts of ROS. This oxidative storm damages the beta-cells, reducing their ability to make insulin. This creates a vicious cycle: high sugar causes oxidative stress, which kills insulin-producing cells, which leads to even higher sugar.
4. Autoimmunity and Chronic Fatigue
Oxidative stress is a trigger for the immune system. When cells are damaged by free radicals, they release “Danger Associated Molecular Patterns” (DAMPs). These signals put the immune system on high alert, potentially triggering or exacerbating autoimmune conditions where the body attacks itself.
Furthermore, in conditions like Chronic Fatigue Syndrome, we often see the mitochondria shutting down energy production to protect the cell from further oxidative damage—a state known as the “Cell Danger Response.” You feel tired because your cells have literally decided it is safer to hibernate than to run a dirty engine.
Measuring the Invisible: Don’t Guess, Test Your Oxidative Stress
You can’t feel oxidative stress until the damage is done. And unfortunately, your standard annual blood panel won’t show it. Your doctor might check your cholesterol or blood sugar, but they aren’t checking if your cells are rusting.
At Agami Health, we believe in looking under the hood. To truly assess your “rust” levels, we use advanced functional markers that offer a window into your cellular health.

1. 8-OHdG (Urine) – The DNA Damage Marker
What it is: 8-Hydroxy-2′-deoxyguanosine.
Why we test it: When free radicals attack your DNA, your body tries to repair it by cutting out the damaged parts and excreting them. 8-OHdG is that excreted debris.
Interpretation: A high level here is a “Red Alert.” It means your genetic material is currently taking a hit. Elevated levels are strongly correlated with cancer risk and accelerated biological aging.
2. F2-Isoprostanes – The Lipid Peroxidation Marker
What it is: A gold-standard marker for damaged fats.
Why we test it: Remember how the brain and cell membranes are made of fat? When those fats oxidize (go rancid), they form F2-Isoprostanes.
Interpretation: This is arguably the most accurate marker of oxidative stress in the body. High levels indicate that your cell membranes are being compromised, which affects how cells talk to each other and how nutrients get in.
3. Oxidized LDL – The Vascular Risk Marker
What it is: A specific measure of the “rusted” cholesterol particles.
Why we test it: As mentioned above, total cholesterol tells us very little. Oxidized LDL tells us if your cholesterol is actively contributing to plaque formation.
4. Glutathione Levels – The Reserve Tank
What it is: Your body’s “Master Antioxidant”.
Why we test it: We need to know not just how much damage is happening, but how much reserve capacity you have to fight it. Low glutathione suggests your cleaning crew is overwhelmed.
The Strategy: The Agami Protocol for Rust-Proofing
So, how do we fix it? The goal isn’t to eliminate ROS entirely (remember, we need the signal), but to restore Redox Homeostasis—the perfect balance between production and elimination.
Pillar 1: Stop Fuelling the Fire (Reduce Production)
The first step is removing the sources that are causing your engine to smoke.
- Toxin Avoidance: We live in a chemical soup. Plastics (phthalates), heavy metals, and pesticides are all “pro-oxidants.” Simple swaps like glass containers instead of plastic, filtering your water, and choosing organic can significantly lower your oxidative load.
- Glucose Control: High blood sugar is rocket fuel for oxidative stress. Keeping your blood sugar stable (avoiding massive spikes) is perhaps the single most powerful thing you can do. Using a Continuous Glucose Monitor (CGM) can be an eye-opening tool here.
Pillar 2: Upgrade the Engine (Mitochondrial Health)
If your mitochondria are leaking too many electrons, we need to repair or replace them.
- Urolithin A & Mitophagy: Over time, our mitochondria get old and dysfunctional. Mitophagy is the process of recycling these engines to make way for new efficient models. Unfortunately, many of us lose the ability to produce Urolithin A (a gut metabolite) as we age. Supplementation is showing promise in human trials for activating this renewal process.
- CoQ10 (Ubiquinol): This molecule is a crucial carrier in the electron transport chain. Levels drop naturally with age, but also plummet if you are taking statins. Supplementing with the active form (Ubiquinol) can help “plug the leaks” in the electron chain.
- PQQ (Pyrroloquinoline Quinone): While CoQ10 helps the engine run, PQQ helps you build new engines (mitochondrial biogenesis).
Pillar 3: Activate the Internal Defense (Nrf2)
Instead of taking a pill that is an antioxidant (like Vitamin C), it is far more effective to take compounds that switch on your body’s own antioxidant factory. This is done by activating a genetic pathway called Nrf2.

- Sulforaphane: Found in cruciferous vegetables (and concentrated in broccoli sprouts), this is one of the most potent Nrf2 activators known to science. It doesn’t just neutralize one free radical; it turns on the genes that produce glutathione, protecting you for days.
- Curcumin & Resveratrol: These polyphenol compounds also help flip the Nrf2 switch. However, bioavailability is key—standard turmeric powder is poorly absorbed, so lipid-based formulations are often required.
- Astaxanthin: This is the pigment that makes salmon pink. It is a unique antioxidant that can span the entire cell membrane, protecting it from the inside out. It is often called “nature’s sunscreen” for its ability to protect skin from UV-induced oxidative stress.
Pillar 4: Hormetic Therapies
Paradoxically, sometimes the best way to fight oxidative stress is to cause a little bit of it—on purpose.
- Hyperbaric Oxygen Therapy (HBOT): By breathing 100% oxygen under pressure, we dramatically increase oxygen levels in the tissue. This creates a transient spike in ROS. The body responds to this “shock” by upregulating its antioxidant defenses and mobilizing stem cells.
- Ozone Therapy: Similar to HBOT, ozone is a “pro-oxidant” therapy. It introduces a controlled oxidative stressor that triggers a net anti-inflammatory response, improving oxygen utilization and circulation.
Conclusion
Aging might be inevitable, but the rate at which you “rust” is not.
When we view health through the lens of oxidative stress, we stop chasing symptoms and start addressing the root cause of cellular decline. It explains why a person can look 40 at age 60, or why another might suffer from chronic fatigue in their 30s.
The strategy is not about flooding your body with generic vitamins. It is about precision. It starts with measuring your baseline—are you rusting? If so, where? Is it your DNA or your cell membranes? Once we know the target, we can deploy the right tools: cleaning up the environment, fixing the mitochondrial engines, and activating your own powerful internal defenses.
You only get one vehicle for this journey of life. It’s worth keeping the engine clean.